
Chemistry, 24.10.2019 03:00 morganlynn18
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (provides a -, the conjugate base of ha). pk a for ha is 6.23. how many moles of a - will be present in the mixture after addition of 0.20 ml 0.010 m naoh? (suggestion: start by calculating the moles of everything in the mixture.) enter the numerical value, without units, to at least 2 significant figures. you may use e-format scientific notation, e. g., 1.23x10 -4 would be entered as 1.23e-4. (note that the acid and base do not add up to 20.00 ml -- water must be added. that does not affect how you answer the question.)

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
You have prepared a buffer by combining 5.00 ml 0.010 m ha (weak acid) and 3.00 ml 0.010 m naa (prov...
Questions

History, 22.04.2021 05:50


Chemistry, 22.04.2021 05:50

Mathematics, 22.04.2021 05:50


Physics, 22.04.2021 05:50

Mathematics, 22.04.2021 05:50

Biology, 22.04.2021 05:50

Health, 22.04.2021 05:50

Mathematics, 22.04.2021 05:50

Biology, 22.04.2021 05:50

Mathematics, 22.04.2021 05:50


Biology, 22.04.2021 05:50

Chemistry, 22.04.2021 05:50



English, 22.04.2021 05:50


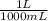 = 5x10⁻³ L of HA
= 5x10⁻³ L of HA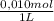 = 5x10⁻⁵ mol of HA
= 5x10⁻⁵ mol of HA

