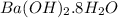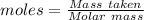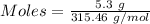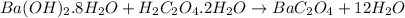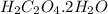Chemistry, 24.10.2019 03:00 austinmontgomep7foxp
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have been assigned to prepare barium oxalate by reacting barium hydroxide octahydrate (ba(oh)2·8h2o) with oxalic acid dihydrate (h2c2o4·2h2o). find the number of grams of oxalic acid dihydrate that would be required to completely react with 5.3 g of barium hydroxide octahydrate. use correct significant figures. do not include a unit with your answer or it will be counted wrong.
h2c2o4·2h2o 126.07 g/mol
h2c2o4 90.03 g/mol
ba(oh)2·8h2o 315.46 g/mol
ba(oh)2 171.34 g/mol

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 23.06.2019 04:00
Which method would be best to separate a mixture of sand and gravel
Answers: 1

Chemistry, 23.06.2019 05:00
Match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) amount of product predicted to be produced by the given reactants theoretical yield c) reactant that can produce more of the product
Answers: 3
You know the right answer?
Barium oxalate is used as a colorant to produce the green color in fireworks. imagine that you have...
Questions

Mathematics, 10.05.2021 20:20

Mathematics, 10.05.2021 20:20



Mathematics, 10.05.2021 20:20


Mathematics, 10.05.2021 20:20



Mathematics, 10.05.2021 20:20

Mathematics, 10.05.2021 20:20









Mathematics, 10.05.2021 20:20