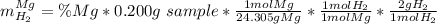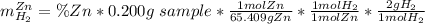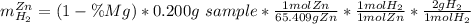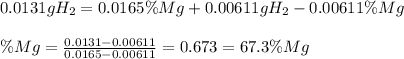Chemistry, 24.10.2019 04:00 sierravick123owr441
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c and 1 atm. the magnesium and zinc are treated with dilute sulfuric acid to produce hydrogen gas according to the following balanced reactions: mg (s) + h2so4 (aq) → mg2+ (aq) + so42– (aq) + h2 (g) zn (s) + h2so4 (aq) → zn2+ (aq) + so42– (aq) + h2 (g) the hydrogen gas is then completely combusted in the same vessel to form water as a product. the vessel is then cooled back to 25 °c (assume that all the water condenses) and the pressure is found to be 0.95 atm. what is the mass percentage of magnesium in the mixture?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 22:30
What is a number added in front of a formula in order to balance the equation
Answers: 1

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3
You know the right answer?
A0.200 g sample of magnesium and zinc is placed in a rigid 1 l vessel containing dry air at 25 °c an...
Questions


Chemistry, 03.10.2021 04:20



Arts, 03.10.2021 04:20


Business, 03.10.2021 04:20

Arts, 03.10.2021 04:20

Mathematics, 03.10.2021 04:20

Physics, 03.10.2021 04:20



Mathematics, 03.10.2021 04:20


Physics, 03.10.2021 04:20

English, 03.10.2021 04:20

Biology, 03.10.2021 04:20



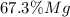
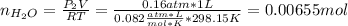
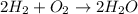
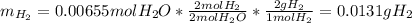
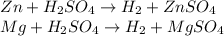
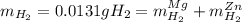
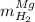 accounts for the hydrogen yielded by the magnesium and
accounts for the hydrogen yielded by the magnesium and 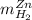 by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below:
by the zinc which are computed in terms of the stoichiometry and the initial sample's composition as shown below: