
Chemistry, 24.10.2019 17:43 odetteerivera
In the process for the manufacture of chlorine by direct oxidation of hcl with air over a catalyst to form cl2 and h2o (only), the exit product is composed of mole fractions of cl2 (21.4%), h2o (21.4%), and n2 (45.0%) – the remainder is hcl and o2. calculate and /or identify: (a) the limiting reactant? (b) the percentage excess reactant? (c) the degree of completion of the reaction? (d) the extent of reaction?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:00
Animals that reproduce sexually either do it through external or internal fertilization. read the following statement and decide if it is true or false. birds reproduce through external reproduction which is because the female will then be able to protect the egg.
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement accurately represents the arrangement of electrons in bohr’s atomic model?
Answers: 2
You know the right answer?
In the process for the manufacture of chlorine by direct oxidation of hcl with air over a catalyst t...
Questions

Biology, 10.09.2020 02:01


Mathematics, 10.09.2020 02:01


Mathematics, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

English, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Biology, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

English, 10.09.2020 02:01

Business, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

Physics, 10.09.2020 02:01

Biology, 10.09.2020 02:01

Biology, 10.09.2020 02:01

Physics, 10.09.2020 02:01


Biology, 10.09.2020 02:01

Mathematics, 10.09.2020 02:01

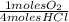 = 0,10725 moles of O₂
= 0,10725 moles of O₂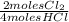 = 0,2145 moles of Cl₂ that are the same than moles of H₂O.
= 0,2145 moles of Cl₂ that are the same than moles of H₂O.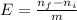
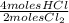 = 0,001 moles of HCl. As initial moles are 0,429 and stoichiometric coefficient is 4, extent of reaction is 0,107
= 0,001 moles of HCl. As initial moles are 0,429 and stoichiometric coefficient is 4, extent of reaction is 0,107

