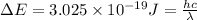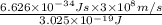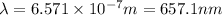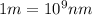The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
e= -ry/n^2
in this equation ry stands for the rydberg energy, and n stands for the principal quantum number of the orbital that holds the electron.
calculate the wavelength of the line in the absorption line spectrum of hydrogen caused by the transition of the electron from an orbital with n=2 to an orbital with n=3.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:00
Which object forms when a supergiant runs out of fuel? a red giant a black hole a white dwarf a neutron star
Answers: 1

Chemistry, 21.06.2019 21:00
Which statement describes evidence of a chemical reaction? a) ice melting eliminate b) water boiling c) lighting a match d) grape juice freezing
Answers: 3

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following is not one of the steps in the scientific method a. hypothesize b. summarize c. analyze d. familiarize
Answers: 3
You know the right answer?
The energy e of the electron in a hydrogen atom can be calculated from the bohr formula:
Questions

Mathematics, 02.07.2019 07:00

Mathematics, 02.07.2019 07:00

Mathematics, 02.07.2019 07:00


English, 02.07.2019 07:00




Mathematics, 02.07.2019 07:00







Mathematics, 02.07.2019 07:00

Mathematics, 02.07.2019 07:00

Mathematics, 02.07.2019 07:00


English, 02.07.2019 07:00

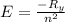

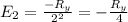
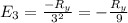
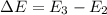 :
: