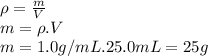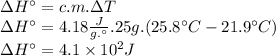Chemistry, 25.10.2019 02:43 hayleymckee
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and water separated by a thin plastic divider. when the divider is broken, the ammonium nitrate dissolves according to the following endothermic reaction: nh4no3(> nh4 ^+(aq) + no3^-(aq). in order to measure the enthalpy change for this reaction, 1.25 g of nh4no3 is dissolved in enough water to make 25.0 ml of solution. the initial temperature is 25.8 degrees c and the final temperature (after the solid dissolves) is 21.9 degrees c. calculate the change in enthalpy for the reaction. (use 1.0g/ml as the density of the solution and 4.18 j/g . degrees c as the specific heat capacity.)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:50
Which statement describes how phase changes can be diagrammed as a substance is heated? the phase is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the phase is on the x-axis. the time is on the y-axis and the temperature is on the x-axis. the temperature is on the y-axis and the time is on the x-axis.
Answers: 1


Chemistry, 22.06.2019 20:50
What is the vapor pressure of a solution with a benzene to octane?
Answers: 2

Chemistry, 22.06.2019 21:40
A5 mole sample of liquid acetone is converted to a gas at 75.0°c. if 628 j are required to raise the temperature of the liquid to the boiling point, 15.600 kj are required to evaporate the liquid, and 712 j are required to raise the final temperature to 75.0°c, what is the total energy required for the conversion?
Answers: 3
You know the right answer?
Instant cold packs, often used to ice athletic injuries on the field, contain ammonium nitrate and w...
Questions



Mathematics, 31.07.2019 07:50




Mathematics, 31.07.2019 07:50





Physics, 31.07.2019 07:50

Biology, 31.07.2019 08:00


History, 31.07.2019 08:00


Biology, 31.07.2019 08:00