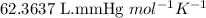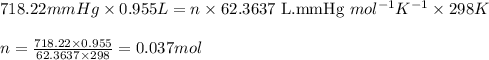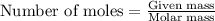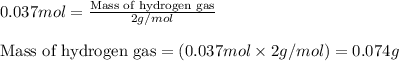Chemistry, 25.10.2019 04:43 allytrujillo20oy0dib
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is filed down in several spots (so that the hydrochloric acid can get to the zinc). the reaction between the acid and the zinc is as follows: 2h+(aq)+zn(s)→h2(g)+zn2+(aq). when the zinc in a certain penny dissolves, the total volume of gas collected over water at 25 ∘c was 0.955 l at a total pressure of 742 mmhg .

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 17:40
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 21:30
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1
You know the right answer?
The zinc within a copper-plated penny will dissolve in hydrochloric acid if the copper coating is fi...
Questions

History, 30.05.2020 16:57

Biology, 30.05.2020 16:57

Mathematics, 30.05.2020 16:57

Mathematics, 30.05.2020 16:57

Business, 30.05.2020 16:57


Mathematics, 30.05.2020 16:57


Mathematics, 30.05.2020 16:57


History, 30.05.2020 16:57

Chemistry, 30.05.2020 16:57





Mathematics, 30.05.2020 16:57



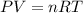
![25^oC=[25+273]K=298K](/tpl/images/0345/5919/df1f6.png)