Determine the ph of a 0.500 m hno2 solution. ka of hno2 is 4.6 * 10-4.
a. 1.82
b....


Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
Asyringe contains 56.05 ml of gas at 315.1 k. what volume will that gas occupy if the temperature is increased to 380.5 k? a) 12.41 b) 46.42 c) 67.68 d) 81.74
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 16:30
Asample of freon gas has a volume of 2.23 liters, a pressure of 4.85 kpa, and a temperature of -1.36°c. calculate the volume at a pressure of 1.38 kpa and a temperature of 5.5°c. (show work)
Answers: 1

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
Questions




English, 22.10.2020 01:01


History, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Advanced Placement (AP), 22.10.2020 01:01



Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01


Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01


Health, 22.10.2020 01:01

Mathematics, 22.10.2020 01:01

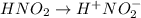
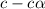
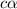
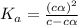
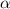 = ?
= ?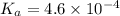
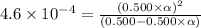
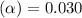
![[H^+]=c\times \alpha](/tpl/images/0346/2002/4fc41.png)
![[H^+]=0.500\times 0.030=0.015](/tpl/images/0346/2002/28636.png)
![pH=-log[H^+]](/tpl/images/0346/2002/15713.png)
![pH=-log[0.015]=1.82](/tpl/images/0346/2002/45771.png)
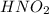 solution is 1.82
solution is 1.82

