
Chemistry, 25.10.2019 17:43 SuperWoman9172
Asample of gas contains 0.1900 mol of co(g) and 0.1900 mol of no(g) and occupies a volume of 22.0 l. the following reaction takes place: 2co(g) + 2no(g)2co2(g) + n2(g) calculate the volume of the sample after the reaction takes place, assuming that the temperature and the pressure remain constant.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 11:40
Enzymes affect the reactions in living cells by changing the
Answers: 3

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1


Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3
You know the right answer?
Asample of gas contains 0.1900 mol of co(g) and 0.1900 mol of no(g) and occupies a volume of 22.0 l....
Questions




Social Studies, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00



Mathematics, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00


Mathematics, 09.02.2021 01:00

Mathematics, 09.02.2021 01:00




History, 09.02.2021 01:00

Social Studies, 09.02.2021 01:00


Mathematics, 09.02.2021 01:00

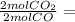 0.1900 mol CO₂
0.1900 mol CO₂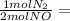 0.095 mol N₂
0.095 mol N₂

