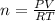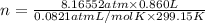Chemistry, 25.10.2019 19:43 nschavez123
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the tire is 860 ml , that it is filled to a total pressure of 120 psi , and that the temperature is 26 ∘c. also, assume an average molar mass for air of 28.8 g/mol.
a) calculate the mass of air in an air filled tire.
b) calculate the mas of helium in a helium-filled tire.
c) what is the mass difference between the two?

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1

Chemistry, 22.06.2019 14:00
Which of the following is true about a carbonated soft drink? . the carbon dioxide is the solvent, and water is the solute.. the water is the solution, and carbon dioxide is the solvent.. the carbon dioxide is the solution, and the water is the solvent.. the water is the solvent, and the carbon dioxide is the solute.. .
Answers: 1

Chemistry, 23.06.2019 02:20
Why dose heating increase the speed at which a solution dissolved in water
Answers: 1

You know the right answer?
Olympic cyclists fill their tires with helium to make them lighter. assume that the volume of the ti...
Questions


Biology, 26.08.2019 01:30



Geography, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30



Mathematics, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30

History, 26.08.2019 01:30

Mathematics, 26.08.2019 01:30



Mathematics, 26.08.2019 01:30


English, 26.08.2019 01:30