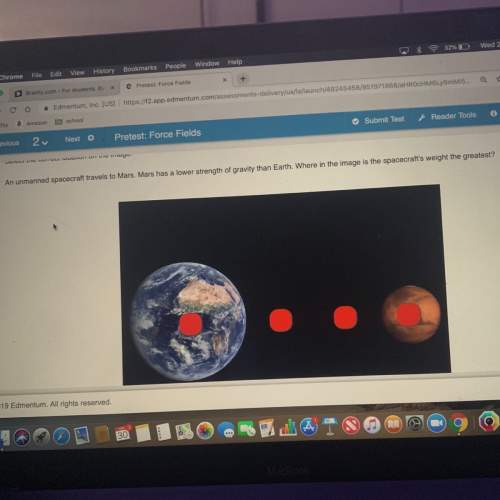
Using the following portion of the activity series for oxidation half reactions
k(s) → k+ (aq)...

Chemistry, 25.10.2019 20:43 blakemccain1928
Using the following portion of the activity series for oxidation half reactions
k(s) → k+ (aq) + e-
al(s) → al3+ (aq) + 3e-
fe(s) → fe2+ (aq) + 2e-
sn(s) → sn2+ (aq) + 2e-
determine which reaction will occur.
a) al3+(aq) with fe(s)
b) al(s) with sn(s)
c) k+(aq) with fe2+(aq)
d) k(s) with sn2+(aq)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:50
2points why do scientists need governmental funding? o a. government politicians ask all the important scientific questions. o b. scientists have to pay taxes to the government on the money they make. o c. the cost of doing scientific research can be very high. o d. the government is controlled by scientists. submit
Answers: 3


Chemistry, 23.06.2019 07:00
Which of the following statements is true? an atom consists of protons, electrons, and neutrons.an atom consists of protons and neutrons.an atom consists of electrons bonded to one another.an atom consists of protons bonded to one another.
Answers: 1

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Questions

Mathematics, 02.12.2021 20:40

Health, 02.12.2021 20:40

Mathematics, 02.12.2021 20:40

Chemistry, 02.12.2021 20:40



Biology, 02.12.2021 20:40

English, 02.12.2021 20:40

Mathematics, 02.12.2021 20:40









English, 02.12.2021 20:40


Computers and Technology, 02.12.2021 20:40