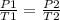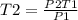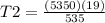Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:00
How does kepler second law of planetary motion overthrow one of the basic beliefs of classical astronomy
Answers: 1

Chemistry, 22.06.2019 20:10
What would happen to a volleyball left outside in the winter? o o o o a. it would expand. b. it would lose air. c. it would shrink. d. it would explode.
Answers: 2
You know the right answer?
Using the same sample of gas (p1 = 535 torr , t1 = 19 ∘c ), we wish to change the pressure to 5350 t...
Questions

Mathematics, 07.05.2021 18:20


Mathematics, 07.05.2021 18:20




History, 07.05.2021 18:20



Biology, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20

Mathematics, 07.05.2021 18:20




Mathematics, 07.05.2021 18:20