
Chemistry, 25.10.2019 22:43 sarahhans6711
In the reaction of aluminum and iron(iii) oxide to form iron and aluminum oxide, δh is -850 kj. how many grams of aluminum oxide are formed when 350 kj of heat are released? 2 al (s) + fe2o3 (s) → 2 fe (s) + al2o3 (s) δh= -850 kj

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 22:30
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 22.06.2019 08:00
What are the similarities of physical and chemical change ?
Answers: 1

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
In the reaction of aluminum and iron(iii) oxide to form iron and aluminum oxide, δh is -850 kj. how...
Questions

English, 18.01.2021 14:00

English, 18.01.2021 14:00

Chemistry, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

English, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

Chemistry, 18.01.2021 14:00


Mathematics, 18.01.2021 14:00



Mathematics, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00



Health, 18.01.2021 14:00


Biology, 18.01.2021 14:00

Chemistry, 18.01.2021 14:00

Mathematics, 18.01.2021 14:00

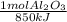 = 0,412 moles of Aluminium oxide.
= 0,412 moles of Aluminium oxide.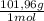 = 41,98 g
= 41,98 g

