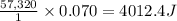Chemistry, 26.10.2019 00:43 tmanpierce
Astudent enters the lab and conducts part a of the experiment. the student uses 32.00 ml of 2.202 m hcl, and adds naoh in excess as instructed. if the δh of the neutralization reaction is known to be -57,320 j/mol h2o, what is the total theoretical heat released (in joules)?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 23:00
Why are the trends and exceptions to the trends in ionization energy observed?
Answers: 1

Chemistry, 22.06.2019 04:00
Tin has ten stable isotopes. the heaviest, 124sn, makes up 5.80% of naturally occuring tin atoms. how many atoms of 124sn are present in 82.0 g of naturally occurring tin? what is the total mass of the 124sn atoms in this sample?
Answers: 3

Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
Astudent enters the lab and conducts part a of the experiment. the student uses 32.00 ml of 2.202 m...
Questions

Mathematics, 21.04.2021 21:40

Mathematics, 21.04.2021 21:40

Mathematics, 21.04.2021 21:40

Business, 21.04.2021 21:40


World Languages, 21.04.2021 21:40

Mathematics, 21.04.2021 21:40

Mathematics, 21.04.2021 21:40


English, 21.04.2021 21:40

Mathematics, 21.04.2021 21:40


Mathematics, 21.04.2021 21:40





Biology, 21.04.2021 21:40


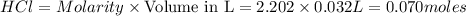
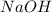 is in excess ,
is in excess , 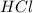 is the limiting reagent as will decide the amount of product formed and energy released.
is the limiting reagent as will decide the amount of product formed and energy released.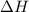 = -57,320 J/mol
= -57,320 J/mol