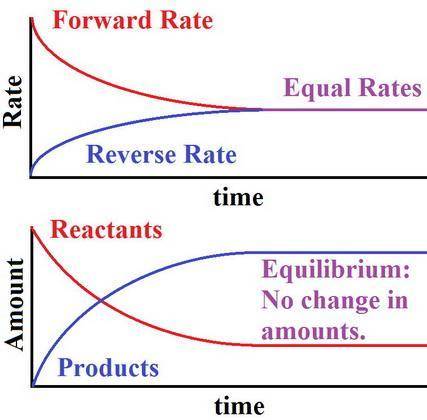
At chemical equilibrium, the amount of (product and reactant is zero, product and reactant remains constant, product is zero) because (all molecules have the same activation energy, an equal number of molecules take part in the reaction, the rates of the forward and reverse reactions are equal)

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1
You know the right answer?
At chemical equilibrium, the amount of (product and reactant is zero, product and reactant remains c...
Questions




Mathematics, 03.02.2021 03:40





Mathematics, 03.02.2021 03:40

Mathematics, 03.02.2021 03:40


Mathematics, 03.02.2021 03:40

Mathematics, 03.02.2021 03:40

English, 03.02.2021 03:40

Social Studies, 03.02.2021 03:40


Mathematics, 03.02.2021 03:40


Chemistry, 03.02.2021 03:40

Mathematics, 03.02.2021 03:40