
Chemistry, 27.10.2019 02:43 Queenofpizza
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74.92 g.)

Answers: 2
Another question on Chemistry



Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 11:00
Acompound is isolated from the rind of lemons that is found to be 88.14% carbon and 11.86% hydrogen by mass how many grams of c and h?
Answers: 2
You know the right answer?
Determine the mass in grams of 3.00 × 10²¹ atoms of arsenic. (the mass of one mole of arsenic is 74....
Questions

Biology, 16.10.2019 21:10

Arts, 16.10.2019 21:10


History, 16.10.2019 21:10


Social Studies, 16.10.2019 21:10




History, 16.10.2019 21:10





Mathematics, 16.10.2019 21:10





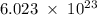 .
.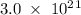 atoms.
atoms.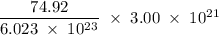 grams
grams

