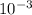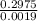Chemistry, 28.10.2019 23:31 crodriguez87
Astudent dissolves 0.2975 g of the unknown weak acid to give 100. ml solution. s/he obtains the following titration curve for his/her best titration. at the equivalence point, 19.0 ml of 0.1000 m naoh was added.(a) calculate the molar mass of the acid. g/mol(b) what is the pka of the acid?

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

You know the right answer?
Astudent dissolves 0.2975 g of the unknown weak acid to give 100. ml solution. s/he obtains the foll...
Questions


Mathematics, 19.05.2021 16:00

Mathematics, 19.05.2021 16:00


Mathematics, 19.05.2021 16:00









Mathematics, 19.05.2021 16:00

Mathematics, 19.05.2021 16:00

Mathematics, 19.05.2021 16:00


Social Studies, 19.05.2021 16:00


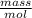 .....................1
.....................1