
Chemistry, 29.10.2019 00:31 ghostshadow1
For parts a and b, explain why the substances follow (or do not follow) the law of multiple proportions. take m(o) = 16 g/mol, m(mn) = 55 g/mol, and m(i) = 127 g/mol and derive the empirical formulae of all mentioned compounds. part a. sample a of manganese (mn) oxide contains 330 mg of mn and 144 mg of o, sample b — 330 mg of mn and 336 mg of o. part b. sample a of iodine (i) oxide contains 508 mg of i and 128 mg of o, sample b — 508 mg of i and 144 mg of o.
2. part a. calculate the mass of barium chloride dihydrate required to prepare a 1.500 l solution of 0.1500 mol/l ba2+. m(bacl2·2h2o) = 244.26 g/mol. part b. calculate the mass of copper(ii) sulfate pentahydrate required to prepare a 2.500 l solution of 0.2500 mol/l cu2+. m(cuso4·5h2o) = 249.69 g/mol.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 04:00
Asample of aluminum foil contains 8.60 × 1023 atoms. what is the mass of the foil?
Answers: 1

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:00
What is the lowest number energy level where a d sublevel is found
Answers: 1
You know the right answer?
For parts a and b, explain why the substances follow (or do not follow) the law of multiple proporti...
Questions




Mathematics, 11.10.2019 14:30

Biology, 11.10.2019 14:30





English, 11.10.2019 14:30


Mathematics, 11.10.2019 14:30



Social Studies, 11.10.2019 14:30

Health, 11.10.2019 14:30

Biology, 11.10.2019 14:30


Mathematics, 11.10.2019 14:30

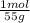 = 6x10⁻³ moles
= 6x10⁻³ moles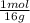 = 9x10⁻³ moles
= 9x10⁻³ moles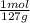 = 4x10⁻³ moles
= 4x10⁻³ moles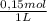 ×
×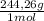 = 54,96g of BaCl₂.2H₂O
= 54,96g of BaCl₂.2H₂O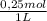 ×
×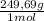 = 156,1g of CuSO₄.5H₂O
= 156,1g of CuSO₄.5H₂O

