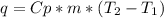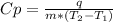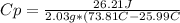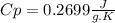Chemistry, 29.10.2019 01:31 thebrain1345
The amount of heat (q) gained or lost by a substance (with mass m) as its temperature changes (δt) depends on its specific heat capacity (cp) according to the following equation. q = cp ✕ m ✕ δt the quality of industrial diamonds is determined in part by measuring the specific heat capacity, which is 0.5091 j/g·k for pure diamond. if the absorption of 26.21 j of heat by a 2.03 g diamond sample of unknown purity causes its temperature to rise from 25.99°c to 73.81°c, is the diamond sample pure? explain your reasoning.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 16:00
What rule is used to determine how many covalent bonds an element can form? a. the number of covalent bonds is equal to six c the number of covalent bonds is equal to five minus the group number plus the group number b. the number of covalent bonds is equal to eight d. none of the above minus the group number select the best answer from the choices provided
Answers: 2


Chemistry, 23.06.2019 13:00
Which of the following statements is true about both nuclear fusion and nuclear fission? they occur in the sun. heavy atoms are split. two light nuclei combine. some mass changes into energy.
Answers: 1

Chemistry, 23.06.2019 14:00
How are casts formed by decaying organisms? organisms turn into rock over time. organisms leave carbon residue on a rock. organisms leave impressions in sediment that hardens into rock. impressions left by organisms are filled in with sediment that hardens into rock.
Answers: 2
You know the right answer?
The amount of heat (q) gained or lost by a substance (with mass m) as its temperature changes (δt) d...
Questions


Mathematics, 25.08.2021 04:40




Mathematics, 25.08.2021 04:40

Computers and Technology, 25.08.2021 04:40

Biology, 25.08.2021 04:50

History, 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

English, 25.08.2021 04:50


Social Studies, 25.08.2021 04:50

History, 25.08.2021 04:50


Business, 25.08.2021 04:50



Mathematics, 25.08.2021 04:50

Mathematics, 25.08.2021 04:50

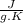 and it is different from the specific heat capacity found that is 0.2699
and it is different from the specific heat capacity found that is 0.2699