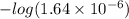Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. dissolved co2 satisfies the equilibrium equation co2(g) co2(aq) k=0.032 the acid dissociation constants listed in most standard reference texts for carbonic acid actually apply to dissolved co2. for a co2 partial pressure of 1.9x10-4 bar in the atmosphere, what is the ph of water in equilibrium with the atmosphere? (for carbonic acid ka1 = 4.46x10-7 and ka2 = 4.69x 10-11).

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 19:30
Si una estrella no tiene paralaje medible, ¿qué puedes inferir?
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 09:00
Which process does not require the presence of a physical substance in order to transfer heat? air in the atmosphere is heated by the ground. this warm air then rises, and cooler air falls. this is an example of what type of process? how is conduction different from radiation?
Answers: 1
You know the right answer?
Carbon dioxide dissolves in water to form carbonic acid, which is primarily dissolved co2. dissolved...
Questions

History, 17.06.2020 13:57


Engineering, 17.06.2020 13:57

Chemistry, 17.06.2020 13:57


English, 17.06.2020 13:57

Mathematics, 17.06.2020 13:57

Health, 17.06.2020 13:57

Biology, 17.06.2020 13:57

History, 17.06.2020 13:57



Mathematics, 17.06.2020 13:57


Mathematics, 17.06.2020 13:57

Mathematics, 17.06.2020 13:57


Mathematics, 17.06.2020 13:57

Biology, 17.06.2020 13:57

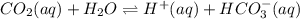
 dissolved as follows.
dissolved as follows.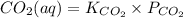
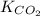 = 0.032 M/atm and
= 0.032 M/atm and 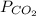 =
= 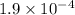 atm.
atm.![[CO_{2}]](/tpl/images/0350/3284/0a7e9.png) will be calculated as follows.
will be calculated as follows.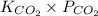
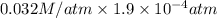
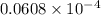
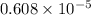
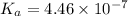
![K_{a} = \frac{[H^{+}]^{2}}{[CO_{2}]}](/tpl/images/0350/3284/deb36.png)
![4.46 \times 10^{-7} = \frac{[H^{+}]^{2}}{0.608 \times 10^{-5}}](/tpl/images/0350/3284/7b4bd.png)
![[H^{+}]^{2}](/tpl/images/0350/3284/724e3.png) =
= 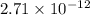
![[H^{+}]](/tpl/images/0350/3284/85507.png) =
= 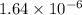
![-log [H^{+}]](/tpl/images/0350/3284/822be.png)