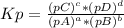Chemistry, 29.10.2019 02:31 Diegosolorzano50
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10-3calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g)write the equilibrium constant expression, kp write the pressures in the following format: (pco2)

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 01:40
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2
You know the right answer?
The following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1...
Questions

English, 08.07.2021 08:50


Social Studies, 08.07.2021 08:50

Mathematics, 08.07.2021 08:50

English, 08.07.2021 08:50

English, 08.07.2021 08:50


Chemistry, 08.07.2021 09:00

Mathematics, 08.07.2021 09:00


Mathematics, 08.07.2021 09:00

Mathematics, 08.07.2021 09:00

Mathematics, 08.07.2021 09:00

Mathematics, 08.07.2021 09:00





Chemistry, 08.07.2021 09:00