
Chemistry, 29.10.2019 04:31 AdanNava699
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
excess ni2+) to convert the cyanide totetracyanonickelate(ii):
4cn- + ni2+ (cn)42-
the excess ni2+ was then titrated with 10.15 ml of0.01307 m acid
(edta). one mole of this reagent reacts with 1 mol ofni2+:
ni2+ + edta4- (edta)2-
ni(cn)42- does not react with edta. if 39.35ml of the 0.01307 m edta was required to react
with 30.10 ml of the original ni2+ solution, calculatethe molarity of cn- in the 12.73 ml cyanide
sample.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2

Chemistry, 22.06.2019 10:40
Which buffer would be better able to hold a steady ph on the addition of strong acid, buffer 1 or buffer 2? explain. buffer 1: a solution containing 0.10 m nh4cl and 1 m nh3. buffer 2: a solution containing 1 m nh4cl and 0.10 m nh3
Answers: 1

Chemistry, 22.06.2019 12:00
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1

Chemistry, 22.06.2019 19:40
What is the wavelength of a 3*10^12 hz infrared wave a 3*10^20m b 1* 10^4m c 3*10^-3m d 1*10^-4 m
Answers: 1
You know the right answer?
Acyanide solution with avolume of 12.73 ml was treated with 25.00 ml of ni2+solution (containing
Questions


Mathematics, 17.10.2019 16:10









History, 17.10.2019 16:10









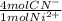 = 1,178x10⁻³ moles of CN⁻
= 1,178x10⁻³ moles of CN⁻

