
Chemistry, 29.10.2019 04:31 21ghostrider21
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zinc sulfide with oxygen gas to produce zinc oxide and sulfur dioxide. 2zns(s) 3o2(g)⟶2zno(s) 2so2(g) when the external pressure is 1.523×105 pa and the temperature is 675 k, the amount of work performed is 850.1 j. calculate how many grams of oxygen are consumed in the reaction.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 03:40
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

Chemistry, 22.06.2019 20:00
Glucose (c6h12o6) is an important biological molecule. (round the answer to nearest hundredth.) what is the percent by mass of carbon in glucose?
Answers: 2

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3
You know the right answer?
The first step in the process used to recover zinc metal from zinc sulfide ore is the reaction of zi...
Questions

Mathematics, 02.07.2019 21:30

Biology, 02.07.2019 21:30


Mathematics, 02.07.2019 21:30


English, 02.07.2019 21:30


Mathematics, 02.07.2019 21:30

Mathematics, 02.07.2019 21:30

Biology, 02.07.2019 21:30


History, 02.07.2019 21:30










 = change in moles of gas = ?
= change in moles of gas = ?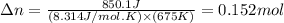
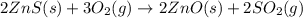
 = 32 g/mol
= 32 g/mol

