Chemistry, 29.10.2019 07:31 acavalieri72
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temperature of the water decreased by 0.93 oc. the heat capacity of the calorimeter is 42.2 j/oc. the density of the water (and the solution) is 1.00 g/ml. the specific heat capacity of the solution is 4.184 j/goc. calculate the enthalpy change for dissolving this salt on a energy per mass basis (units of j/g).

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 23.06.2019 00:30
Nuclear decay is the spontaneous decay of one element into a. an x-ray b. a ray of light c. another element
Answers: 1

You know the right answer?
Consider the dissolution of 2.50 grams of salt xy in 75.0 ml of water within a calorimeter. the temp...
Questions

Mathematics, 20.05.2020 00:59


Biology, 20.05.2020 00:59

History, 20.05.2020 00:59



Social Studies, 20.05.2020 00:59



History, 20.05.2020 00:59

Mathematics, 20.05.2020 00:59

Computers and Technology, 20.05.2020 00:59

Mathematics, 20.05.2020 00:59

Social Studies, 20.05.2020 00:59


Mathematics, 20.05.2020 00:59


English, 20.05.2020 00:59

Chemistry, 20.05.2020 00:59

![q=[q_1+q_2]](/tpl/images/0350/9166/341bc.png)
![q=[c_1\times \Delta T+m_2\times c_2\times \Delta T]](/tpl/images/0350/9166/1d50b.png)
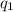 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter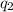 = heat absorbed by the water
= heat absorbed by the water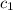 = specific heat of calorimeter =
= specific heat of calorimeter = 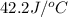
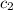 = specific heat of water =
= specific heat of water = 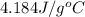
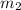 = mass of water =
= mass of water = 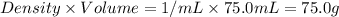
 = change in temperature =
= change in temperature = 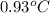
![q=[(42.2J/^oC\times 0.93^oC)+(75.0g\times 4.184J/g^oC\times 0.93^oC)]](/tpl/images/0350/9166/57473.png)
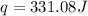
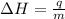
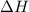 = enthalpy change = ?
= enthalpy change = ?