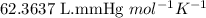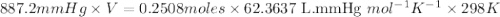Chemistry, 30.10.2019 01:31 scottmichetti
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction include aqueous calcium chloride, liquid water, and gaseous carbon dioxide. calculate the volume of co₂ gas collected over water at 25.0 °c when 25.1 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm hg. the vapor pressure of water at 25.0 °c is 23.8 mm hg.

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 08:30
For each of the compounds below, show that the charges on the ions add up to zero. a. kbr b. cao c. li(2)o d. cacl(2) e. alcl(3)
Answers: 2

Chemistry, 22.06.2019 14:50
The table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of tthe table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 9 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 3

Chemistry, 22.06.2019 22:10
What is the indicator of the number of ions in solution? the amount of conductivity the amount of precipitate the amount of solute added
Answers: 1
You know the right answer?
When solid calcium carbonate is reacted with aqueous hydrochloric acid, the products of the reaction...
Questions

Mathematics, 08.01.2021 03:50



Mathematics, 08.01.2021 03:50


Mathematics, 08.01.2021 03:50



Mathematics, 08.01.2021 03:50




Mathematics, 08.01.2021 03:50



Mathematics, 08.01.2021 04:00




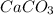 = 100.0869 g/mol
= 100.0869 g/mol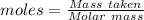
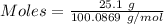
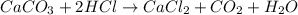
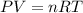
![25^oC=[25+273]K=298K](/tpl/images/0351/9682/df1f6.png)