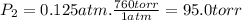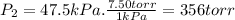Chemistry, 30.10.2019 01:31 alexmoy45p8yd7v
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of the specified gases. bulb a: 150. ml of co(g) at 190. torr bulb b: 300. ml of ar(g) at 0.500 atm bulb c: 750. ml of kr(g) at 75.994 kpa 1. after both stopcocks are opened and the gases allowed to diffuse throughout, what will be the ultimate total pressure? 2. what is the partial pressure of co(g)?
3. what is the mole fraction of co₂(g)?

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 06:00
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2
You know the right answer?
Three glass bulbs, joined by closed stopcocks, have the following volumes and initial pressures of t...
Questions


Social Studies, 30.07.2019 10:30

Biology, 30.07.2019 10:30

Biology, 30.07.2019 10:30



Biology, 30.07.2019 10:30






History, 30.07.2019 10:30

History, 30.07.2019 10:30


Computers and Technology, 30.07.2019 10:30

Mathematics, 30.07.2019 10:30

Mathematics, 30.07.2019 10:30


Mathematics, 30.07.2019 10:30