
Chemistry, 30.10.2019 01:31 musfirahkhurram
Many common weak bases are derivatives of nh3, where one or more of the hydrogen atoms have been replaced by another substituent. such reactions can be generically symbolized as nx3(aq)+h2o(l)⇌hnx3+(aq)+oh−(aq) where nx3 is the base and hnx3+ is the conjugate acid. the equilibrium-constant expression for this reaction is kb=[hnx3+][oh−][nx3] where kb is the base ionization constant. the extent of ionization, and thus the strength of the base, increases as the value of kb increases. ka and kb are related through the equation ka×kb=kw as the strength of an acid increases, its ka value increase and the strength of the conjugate base decreases (smaller kb value). part a if kb for nx3 is 9.0×10−6, what is the poh of a 0.175 m aqueous solution of nx3? express your answer numerically.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:30
A48 g piece of ice at 0.0 ∘c is added to a sample of water at 7.4 ∘c. all of the ice melts and the temperature of the water decreases to 0.0 ∘c. how many grams of water were in the sample?
Answers: 1

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2
You know the right answer?
Many common weak bases are derivatives of nh3, where one or more of the hydrogen atoms have been rep...
Questions



Biology, 29.09.2019 07:30




Biology, 29.09.2019 07:30

History, 29.09.2019 07:30

Mathematics, 29.09.2019 07:30


History, 29.09.2019 07:30






English, 29.09.2019 07:30


Biology, 29.09.2019 07:30

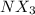 .
.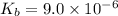
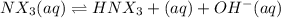
![K_b=\frac{[HNX_3][OH^-]}{[NX_3]}](/tpl/images/0351/9348/b0976.png)
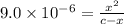
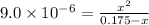

![[HNX_3]=[OH^-]=0.0012505 M](/tpl/images/0351/9348/339f9.png)
![pOH=-\log[OH^-]](/tpl/images/0351/9348/fe336.png)
![pOH=-\log[0.0012505 ]=2.90](/tpl/images/0351/9348/32d39.png)


