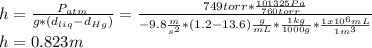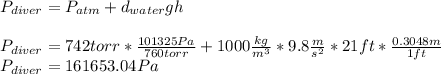Chemistry, 30.10.2019 03:31 jpichardo2021
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercury is 13.6g/ml. what do you predict for the height of a barometer column based on 1-iodododecane, when the atmospheric pressure is 749 torr? what is the pressure, in atmospheres, on the body of a diver if he is 21 ft below the surface of the water when the atmospheric pressure is 742 torr?

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1
You know the right answer?
The compound 1-iodododecane is a nonvolatile liquid with a density of 1.20g/ml. the density of mercu...
Questions

Mathematics, 11.01.2021 14:00


Computers and Technology, 11.01.2021 14:00


Mathematics, 11.01.2021 14:00

Mathematics, 11.01.2021 14:00

Biology, 11.01.2021 14:00

Mathematics, 11.01.2021 14:00

Biology, 11.01.2021 14:00



Mathematics, 11.01.2021 14:00



Mathematics, 11.01.2021 14:00


Social Studies, 11.01.2021 14:00

History, 11.01.2021 14:00

Mathematics, 11.01.2021 14:00

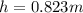
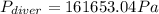
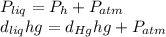
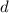 is the density,
is the density, 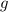 the acceleration of gravity,
the acceleration of gravity, 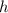 the height and
the height and 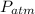 the atmospheric pressure, thus:
the atmospheric pressure, thus: