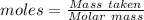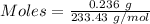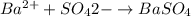Chemistry, 30.10.2019 03:31 putaprincess16
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the solution, a white precipitate forms. the precipitate is filtered and dried and then found to have a mass of 236 mg. what mass of barium was in the original solution? (assume that all of the barium was precipitated out of solution by the reaction.)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 16:30
An atom with 7 protons, 6 neutrons, and 7 electrons has an atomic mass of amu. (enter a whole number.) numerical answers expected! answer for blank 1:
Answers: 3

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1
You know the right answer?
Asolution contains an unknown mass of dissolved barium ions. when sodium sulfate is added to the sol...
Questions



Mathematics, 03.12.2020 19:50


Mathematics, 03.12.2020 19:50


Chemistry, 03.12.2020 19:50


Chemistry, 03.12.2020 19:50

Mathematics, 03.12.2020 19:50




Biology, 03.12.2020 19:50


Social Studies, 03.12.2020 19:50

Arts, 03.12.2020 19:50

Health, 03.12.2020 19:50


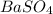 obtained on precipitation = 236 mg
obtained on precipitation = 236 mg