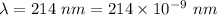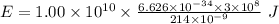Chemistry, 30.10.2019 03:31 lilyella1004
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if there are 1.00×1010 atoms of zinc emitting light in the instrument flame at any given instant, what energy (in joules) must the flame continuously supply to achieve this level of emission? express your answer numerically in joules.
part b during an emission, electrons move from a higher energy orbital to a lower energy orbital. which of the following are valid transitions that produce lines in the emission spectrum of zn?
check all that apply.
1-[ar]4s13d106s1→[ar]4s23d10
2-[ar]4s23d10→[ar]4s23d104p2
3-[ar]4s23d10→[ar]3d10
4-[ar]4s23d10→[ar]4s13d11
5-[ar]3d10→[ar]4s23d10
6-[ar]4s23d10→[ar]4s13d106s1

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 13:30
Why does asexual reproduction result in offspring with identicle genetic variation
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement best describes isomers? a. isomers are alcohols that have the same functional group. b. isomers have at least one carbon-carbon double bond. c. isomers have the same molecular formula but different structural properties.
Answers: 1

Chemistry, 23.06.2019 08:40
A20 liter cylinder of helium at a pressure of 150 atm and a temperature of 27°c is used to fill a balloon at 1.00 atm and 37°c. what is the volume of the balloon? a. 0.14 liters b. 3000 liters c. 2900 liters d. 2400 liters e. 3100 liters
Answers: 1
You know the right answer?
Part a the emission line used for zinc determinations in atomic emission spectroscopy is 214 nm. if...
Questions



Social Studies, 07.12.2021 16:40



Computers and Technology, 07.12.2021 16:40

Mathematics, 07.12.2021 16:40




History, 07.12.2021 16:40

Mathematics, 07.12.2021 16:40

Computers and Technology, 07.12.2021 16:40




Social Studies, 07.12.2021 16:40


Mathematics, 07.12.2021 16:40

Social Studies, 07.12.2021 16:40

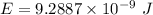
![[Ar]4s^13d^{10}6s^1\rightarrow [Ar]4s^23d^{10}](/tpl/images/0352/1669/c1431.png)
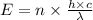
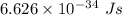
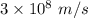
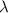 is the wavelength
is the wavelength