Chemistry, 30.10.2019 03:31 richtercatrina16
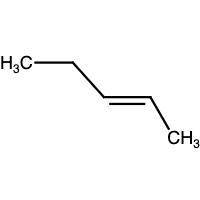
Compound x and compound y are constitutional isomers with the molecular formula c5h10. compound x possesses a carbon-carbon double bond in the trans configuration, while compound y possesses a carbon-carbon double bond that is not stereoisomeric: get answering molecular drawing questions get answering molecular drawing questions. draw the structure of compound x.

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 15:30
How many atoms are in 1.4 mil of phosphorus trifluoride (pf3)
Answers: 3

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 18:30
How many moles of lead are in 1.50 x 10^12 atoms of lead? could you explain the answer as well and not just give it to me i am refreshing for finals and i need to know how to do it
Answers: 3
You know the right answer?
Compound x and compound y are constitutional isomers with the molecular formula c5h10. compound x po...
Questions

Mathematics, 11.06.2021 20:20

Mathematics, 11.06.2021 20:20

Mathematics, 11.06.2021 20:20

Geography, 11.06.2021 20:20

Mathematics, 11.06.2021 20:20



Spanish, 11.06.2021 20:20

History, 11.06.2021 20:20


History, 11.06.2021 20:20


Mathematics, 11.06.2021 20:20

History, 11.06.2021 20:20






Computers and Technology, 11.06.2021 20:20