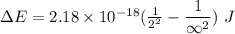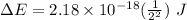Chemistry, 30.10.2019 05:31 htiffany0225
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially in the n =2 energy level. the energy needed to ionize a ground-state hydrogen atom is 2.18 x 10–18 j.

Answers: 1
Another question on Chemistry


Chemistry, 22.06.2019 21:50
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l.s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

You know the right answer?
Calculate the energy, in joules, required to ionize a hydrogen atom when its electron is initially i...
Questions

Mathematics, 03.02.2021 03:00

English, 03.02.2021 03:00

Mathematics, 03.02.2021 03:00

Mathematics, 03.02.2021 03:00

History, 03.02.2021 03:00

Spanish, 03.02.2021 03:00

English, 03.02.2021 03:00




Mathematics, 03.02.2021 03:00

English, 03.02.2021 03:00

Mathematics, 03.02.2021 03:00

Computers and Technology, 03.02.2021 03:00




Mathematics, 03.02.2021 03:00

Mathematics, 03.02.2021 03:00

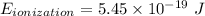
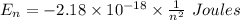
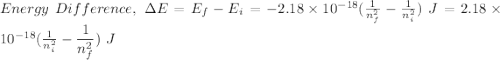
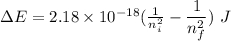
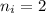 and
and 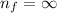 (As the hydrogen has to ionize)
(As the hydrogen has to ionize)