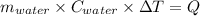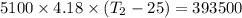Chemistry, 30.10.2019 05:31 mashedpotatoes28
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pressure to form co2(g). all of the heat produced is used to heat a 5100 g bath of liquid water, originally at 25◦c. what is the final temperature of the water bath? the heat of formation of co2(g) is −393.5 kj/mol and the specific heat of water is 4.18 j/g/◦c. answer in units of ◦c

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 02:50
What is the overall order of reaction for rate = k[no]2[o2]
Answers: 3

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 23.06.2019 02:00
Alice did an experiment to find the relationship between the angle at which a ray of light strikes a mirror and the angle at which the mirror reflects the light. she placed a ray box in front of a mirror. she changed the angle at which the light from the ray box struck the mirror and noted the corresponding angle at which the mirror reflected the light. which of the following is the dependent variable in this experiment? the mirror used to reflect the light the ray box used as the source of light angle at which the light from the ray box strikes the mirror angle at which the mirror reflects the light from the ray box
Answers: 2

Chemistry, 23.06.2019 04:00
How many liters of water can be produced from 5.0liters of butane gas at stp, assuming excess oxygen? c4h10(g) + 02(g) → co2 (e) + h2o (g)
Answers: 2
You know the right answer?
One mole of carbon (12.0 g) in the form of crystalline graphite is burned at 25◦c and 1.000 atm pres...
Questions

Mathematics, 04.02.2021 04:00


Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00


Mathematics, 04.02.2021 04:00


Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00


Mathematics, 04.02.2021 04:00

Mathematics, 04.02.2021 04:00






English, 04.02.2021 04:00