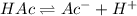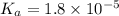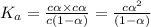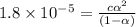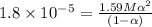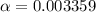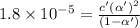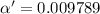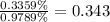Chemistry, 30.10.2019 05:31 skywil8981
Consider that you have two solutions of acetic acid, ka = 1.8x10-5, one solution that is 1.59 m and another solution that is 0.186 m. compare the percent dissociation of acetic acid in these two solutions. what is the ratio of percent dissociation of the 1.59 m solution to the 0.186 m solution? (% dissociation of 1.59 m / % dissociation of 0.186 m) enter your answer numerically to three significant figures.

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 3

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 13:10
Which electron configuration represents the electrons in an atom of sodium in the ground state at stp
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Consider that you have two solutions of acetic acid, ka = 1.8x10-5, one solution that is 1.59 m and...
Questions

Mathematics, 31.08.2021 01:50

History, 31.08.2021 01:50



Mathematics, 31.08.2021 01:50

Mathematics, 31.08.2021 01:50

English, 31.08.2021 01:50




Mathematics, 31.08.2021 01:50


Mathematics, 31.08.2021 01:50


Mathematics, 31.08.2021 01:50


Mathematics, 31.08.2021 01:50



Mathematics, 31.08.2021 01:50