
Chemistry, 30.10.2019 05:31 steven123pink
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and potassium bicarbonate ( mm=100.1154 g/mol ) is dissolved in distilled water. a volume of 31.70 ml of a 0.747 m hcl standard solution is required to titrate the mixture to a bromocresol green end point. calculate the weight percent of potassium carbonate and potassium bicarbonate in the mixture.

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:20
Describing intermolecular forces use the drop down menus to match the type of intermolecular force to its name dipole dipole interactions dipole induced dipole interactions london dispersion forces hydrogen bond van der waals forces done
Answers: 1

Chemistry, 22.06.2019 11:40
Consider this equilibrium: n29) + o2(g) + 2no(c).nitrogen gas and oxygen gas react when placed in a closed container. as the reaction proceeds towards equilibrium, what happens to the rate of thereverse reaction?
Answers: 1

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

You know the right answer?
A2.137 g sample of a solid mixture containing only potassium carbonate ( mm=138.2058 g/mol ) and pot...
Questions

Mathematics, 21.12.2020 18:50

Spanish, 21.12.2020 18:50

English, 21.12.2020 18:50

Mathematics, 21.12.2020 18:50

Mathematics, 21.12.2020 18:50

Physics, 21.12.2020 18:50


Mathematics, 21.12.2020 18:50


Mathematics, 21.12.2020 18:50

Health, 21.12.2020 18:50


Chemistry, 21.12.2020 18:50

Mathematics, 21.12.2020 18:50

Computers and Technology, 21.12.2020 18:50




Spanish, 21.12.2020 18:50

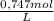 = 0,0237 mol of HCl
= 0,0237 mol of HCl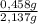 ×100 = 21,4 wt%
×100 = 21,4 wt%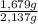 ×100 = 78,6 wt%
×100 = 78,6 wt%

