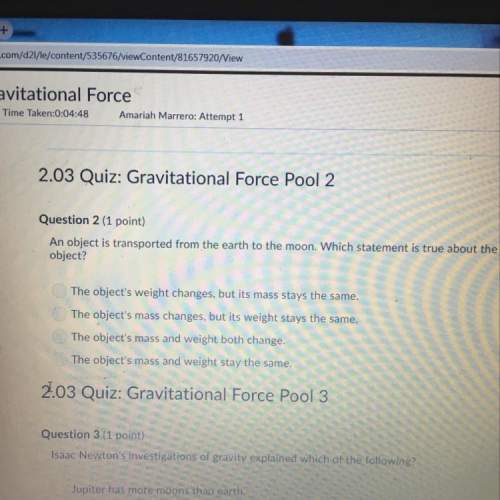
Why are the trends and exceptions to the trends in ionization energy observed? check all that apply.
lonization energy tends to increase down a group because the electrons get farther away from the
nucleus.
b lonization energy tends to increase across a period because the nuclear charge increases.
lonization energy tends to increase across a period because electrons are added to the same main
energy level.
the ionization energies of the elements in group 16 tend to be slightly smaller than the elements in
group 15 because the fourth electron is added to an unfilled p orbital.
the ionization energies of elements in group 13 tend to be lower than the elements in group 2
because the full s orbital shields the electron in the p orbital from the nucleus.

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 11:30
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

You know the right answer?
Why are the trends and exceptions to the trends in ionization energy observed? check all that apply...
Questions


History, 22.10.2020 06:01




History, 22.10.2020 06:01

SAT, 22.10.2020 06:01

Health, 22.10.2020 06:01


Mathematics, 22.10.2020 06:01


Computers and Technology, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01

Geography, 22.10.2020 06:01

Mathematics, 22.10.2020 06:01