48. at 35°c, k = 1.6 x 10-5 for the reaction
2noci(g) = 2no(g) + cl2(g)
calculate the co...

Chemistry, 30.10.2019 22:31 starburst2005
48. at 35°c, k = 1.6 x 10-5 for the reaction
2noci(g) = 2no(g) + cl2(g)
calculate the concentrations of all species at equilibrium
for each of the following original mixtures.
a. 2.0 moles of pure noci in a 2.0-l flask
b. 2.0 moles of no and 1.0 mole of cl2 in a 1.0-l
flask
c. 1.0 mole of noci and 1.0 mole of no in a 1.0-l
flask
d. 3.0 moles of no and 1.0 mole of cl2 in a 1.0-l
flask
e. 2.0 moles of noci, 2.0 moles of no, and 1.0 mole
of cl2 in a 1.0-l flask
f. 1.00 mol/l concentration of all three gases

Answers: 2
Another question on Chemistry


Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 12:30
Write the chemical formula for a compound that is made of an element from group 1 and an element from group 17
Answers: 1

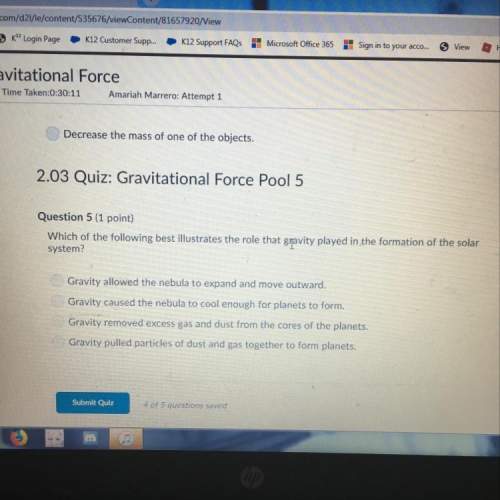
You know the right answer?
Questions

English, 09.07.2019 21:00



Social Studies, 09.07.2019 21:00







Computers and Technology, 09.07.2019 21:10