
Chemistry, 31.10.2019 01:31 CaraRose1887
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both measured at 315 k and 50.0 mmhg), what is the limiting reactant and the theoretical yield of so3? if 187.2 ml of so3 is collected (measured at 315 k and 50.0 mmhg), what is the percent yield for the reaction?

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 19:00
0.66y = 0.9x + 0.48 if y has a value of 108.45 what is the value of x?
Answers: 1

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 23:00
What is the mass of naoh that would have to be added to 500 ml of a solution of 0.20 m acetic acid in order to achieve a ph of 5.0?
Answers: 1
You know the right answer?
Consider the reaction: 2 so2(g)+o2(g)→2 so3(g) if 285.5 ml of so2 reacts with 158.9 ml of o2 (both...
Questions

English, 26.12.2019 04:31

Physics, 26.12.2019 04:31

History, 26.12.2019 04:31

Business, 26.12.2019 04:31

Mathematics, 26.12.2019 04:31

English, 26.12.2019 04:31


Biology, 26.12.2019 04:31


Mathematics, 26.12.2019 04:31

Health, 26.12.2019 04:31





Social Studies, 26.12.2019 04:31

Mathematics, 26.12.2019 04:31


History, 26.12.2019 04:31


 %
%

 moles of
moles of  as follows:
as follows: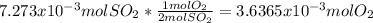
 moles are available in comparison with the
moles are available in comparison with the  moles that completely would react with
moles that completely would react with 

 %
% %
%

