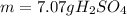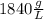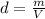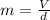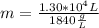Chemistry, 01.11.2019 01:31 miargaree1823
Combustion of coal releases sulfur dioxide into the atmosphere. the following process converts this gas into sulfuric acid, a component of acid rain. 2so2(g) + o2(g) → 2so3(g) so3(g) + h2o(l) → h2so4(aq) if each tonne of coal produces 1.30 × 104 l of sulfur dioxide (measured at stp), what mass of sulfuric acid can result from combustion of each tonne of coal? (1 tonne = 1000 kg)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1


Chemistry, 23.06.2019 04:20
The graph shows one consequence of urban sprawl. how did urban sprawl contribute to the change in biodiversity
Answers: 2

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
Combustion of coal releases sulfur dioxide into the atmosphere. the following process converts this...
Questions


English, 31.08.2020 02:01





Advanced Placement (AP), 31.08.2020 02:01






Mathematics, 31.08.2020 02:01




Mathematics, 31.08.2020 02:01

Computers and Technology, 31.08.2020 02:01

Mathematics, 31.08.2020 02:01

Social Studies, 31.08.2020 02:01