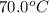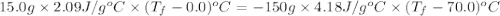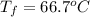15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calorimeter. calculate the final temperature reached, assuming no heat loss or gain from the surroundings. (the specific heat capacity of h2o(l), cs = 4.18 j/gc; h2o(s) h2o(l) h = 6.02 kj/mol)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 15:30
Arock can be broken down into different kinds of substances by physical processes. no chemical reactions are needed to separate different parts of a rock into pure substances. this is because a rock is a(n)
Answers: 1

Chemistry, 22.06.2019 00:00
Substance x has a fixed volume, and the attraction between its particles is strong .substance y had widely spread out particles and can be compressed what can most likely be concluded about these substances
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
15.0 g of ice cubes at 0.0°c are combined with 150. g of liquid water at 70.0°c in a coffee cup calo...
Questions







Mathematics, 18.02.2021 20:50





Business, 18.02.2021 20:50

Mathematics, 18.02.2021 20:50

Spanish, 18.02.2021 20:50


Mathematics, 18.02.2021 20:50




History, 18.02.2021 20:50

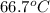
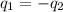
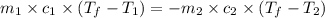
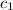 = specific heat of ice =
= specific heat of ice = 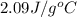
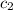 = specific heat of water =
= specific heat of water = 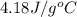
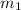 = mass of ice = 15.0 g
= mass of ice = 15.0 g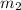 = mass of water = 150 g
= mass of water = 150 g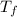 = final temperature = ?
= final temperature = ?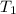 = initial temperature of ice =
= initial temperature of ice = 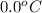
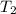 = initial temperature of water =
= initial temperature of water =