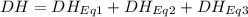At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2(g) δh = –198.9 kj eq. 2: 2 o3(g) → 3 o2(g) δh = –284.6 kj eq. 3: 2 o2(g) → 4 o(g) δh = –990.0 kj use the above data to calculate the heat absorbed (kj) when 2.5 moles of no2(g) is formed at room temperature according to the chemical reaction. do not include units. no(g) + o(g) → no2(g)

Answers: 2
Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 22.06.2019 23:30
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
At room temperature, the following heats of reaction are known: eq. 1: no(g) + o3(g) → no2(g) + o2...
Questions


Medicine, 12.10.2019 00:10



Biology, 12.10.2019 00:10



Medicine, 12.10.2019 00:10



Mathematics, 12.10.2019 00:10


Physics, 12.10.2019 00:10

Mathematics, 12.10.2019 00:10

Geography, 12.10.2019 00:10

French, 12.10.2019 00:10

English, 12.10.2019 00:10



Mathematics, 12.10.2019 00:10