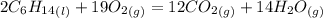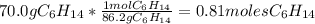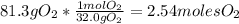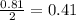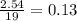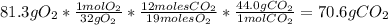Chemistry, 01.11.2019 02:31 twistedgamerhd12
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . suppose 70. g of hexane is mixed with 81.3 g of oxygen. calculate the maximum mass of carbon dioxide that could be produced by the chemical reaction. be sure your answer has the correct number of significant digits.

Answers: 2
Another question on Chemistry


Chemistry, 23.06.2019 00:00
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1

Chemistry, 23.06.2019 16:00
Which question comparing two different metals is best answered through science? a. which metal is easier to use? b. which metal is harder to find? c. which metal is less dense? d. which metal is more valuable?
Answers: 2
You know the right answer?
Problem page liquid hexane will react with gaseous oxygen to produce gaseous carbon dioxide and gase...
Questions


Mathematics, 23.09.2019 18:30

Social Studies, 23.09.2019 18:30




English, 23.09.2019 18:30

Mathematics, 23.09.2019 18:30


History, 23.09.2019 18:30




Computers and Technology, 23.09.2019 18:30

Computers and Technology, 23.09.2019 18:30


History, 23.09.2019 18:30


Mathematics, 23.09.2019 18:30

English, 23.09.2019 18:30