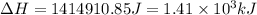Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam at 125 ∘c. the following constants may be useful: cm (ice)=36.57 j/(mol⋅∘c) cm (water)=75.40 j/(mol⋅∘c) cm (steam)=36.04 j/(mol⋅∘c) δhfus=+6.01 kj/mol δhvap=+40.67 kj/mol express your answer with the appropriate units. view available hint(s)

Answers: 1
Another question on Chemistry

Chemistry, 21.06.2019 14:00
A32 year old immigrant from a patriarchal country is giving birth. as she is delivering the baby, she tearfully confesses to her doctor that this is her 4th child and she simply cannot handle any more children. she tells the doctor that her husband refuses to use contraception or allow her to, and she begs her doctor to tie her tubes and not tell her husband. the doctor complies. was hipaa violated? why or why not?
Answers: 3

Chemistry, 22.06.2019 05:30
Liv sheldon given the balanced equation for an organic reaction: c2h2 + 2cl2 → c2h2cl4 this reaction is best classified as *
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1

You know the right answer?
Calculate the amount of energy in kilojoules needed to change 459 g of water ice at −10 ∘c to steam...
Questions

World Languages, 05.02.2020 04:44

History, 05.02.2020 04:44


Social Studies, 05.02.2020 04:44

Mathematics, 05.02.2020 04:44







Biology, 05.02.2020 04:44


Mathematics, 05.02.2020 04:44


History, 05.02.2020 04:44


History, 05.02.2020 04:44


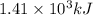
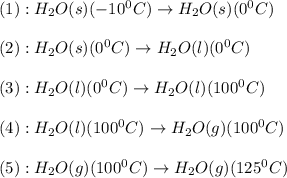
![\Delta H=[n\times c_{ice}\times (T_{final}-T_{initial})]+n\times \Delta H_{fusion}+[n\times c_{water}\times (T_{final}-T_{initial})]+n\times \Delta H_{vap}+[n\times c_{steam}\times (T_{final}-T_{initial})]](/tpl/images/0355/0743/9dcae.png)
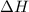 = enthalpy change = ?
= enthalpy change = ?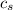 = specific heat of ice =
= specific heat of ice = 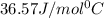
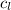 = specific heat of water =
= specific heat of water = 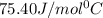
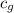 = specific heat of steam =
= specific heat of steam = 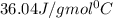
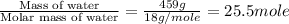
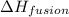 = enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole
= enthalpy change for fusion = 6.01 KJ/mole = 6010 J/mole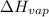 = enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole
= enthalpy change for vaporization = 40.67 KJ/mole = 40670 J/mole![\Delta H=[25.5mole\times 36.57J/mol^0C\times (0-(-10))^0C]+25.5mole\times 6010J/mole+[25.5mole\times 75.40J/mol^0C\times (100-0)^0C]+25.5mole\times 40670J/mole+[25.5mole\times 36.04J/gmol^0C\times (125-100)^0c]](/tpl/images/0355/0743/cfaa1.png)