
Chemistry, 01.11.2019 02:31 luiscastaenda
Uman blood is kept at a typical ph of 7.40 mainly by the carbonic acid–carbonate ion buffer system. the corresponding chemical equation describing the buffer would be: h2co3(aq) h2o(l) ⇌ hco3-(aq) h3o (aq) the appropriate ka value for carbonic acid is 4.3×10-7, so its pka value is 6.37. calculate the [base]/[acid] ratio in human blood. (answer to 3 significant figures, no units)

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 00:20
Use the gizmo to find the concentration of the mystery ch3cooh. use the titrant and indicator shown below perform the titration. what is the titrant volume? titrant analyte indicator titrant volume analyte concentration naoh ch3cooh phenophthalein select one: a. 20.0 ml b. 27.0 ml c. 30.0 ml d. 24.0 ml
Answers: 2

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3

Chemistry, 23.06.2019 05:00
Select the statement that describe chemical properties a. antacid tablets neutralize stomach acid b. helium is the lightest monatomic element c. water freezes at 0 celsius d. mercury is liquid at room temperature
Answers: 3
You know the right answer?
Uman blood is kept at a typical ph of 7.40 mainly by the carbonic acid–carbonate ion buffer system....
Questions

Spanish, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10



Mathematics, 18.03.2021 03:10


Chemistry, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10



Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10

Arts, 18.03.2021 03:10

Mathematics, 18.03.2021 03:10


English, 18.03.2021 03:10


Mathematics, 18.03.2021 03:10

![\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]}](/tpl/images/0355/0720/887f9.png) , is 10.7
, is 10.7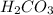 is an weak acid and
is an weak acid and 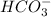 is it's conjugate base.
is it's conjugate base.![pH=pK_{a}(H_{2}CO_{3})+log(\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]})](/tpl/images/0355/0720/8cc41.png)
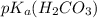 =6.37
=6.37![7.40=6.37+log(\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]})](/tpl/images/0355/0720/a095c.png)
![\frac{[HCO_{3}^{-}]}{[H_{2}CO_{3}]}=10.7](/tpl/images/0355/0720/0e463.png)


