
According to the vsepr model, the arrangement of electron pairs around nh3 and ch4 is a. the same, because in each case there are the same number of electron pairs around the central atom b. different or the same, depending on the conditions leading to maximum repulsion c. different, because in each case there are a different number of atoms around the central atom d. the same, because both nitrogen and carbon are both in the second period e. different, because in each case there are a different number of electron pairs around the central atom

Answers: 1
Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2


Chemistry, 23.06.2019 03:00
The size (radius) of an oxygen molecule is about 2.0 ×10−10m. make a rough estimate of the pressure at which the finite volume of the molecules should cause noticeable deviations from ideal-gas behavior at ordinary temperatures (t= 300k ). assume that deviatons would be noticeable when volume of the gas per molecule equals the volume of the molecule itself.
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
According to the vsepr model, the arrangement of electron pairs around nh3 and ch4 is a. the same, b...
Questions


Mathematics, 24.06.2019 05:50






Mathematics, 24.06.2019 05:50





Chemistry, 24.06.2019 05:50




Mathematics, 24.06.2019 05:50



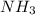 and
and 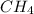 , the number of electron pairs are same but methane has all the four bond pairs where in ammonia, three bond pairs and one lone pair exists. And thus there are repulsion forces possible in between the lone pair and bond pair of electrons thus the arrangement of electron pairs around both the molecules is same or different depending up on the conditions leading to maximum repulsion.
, the number of electron pairs are same but methane has all the four bond pairs where in ammonia, three bond pairs and one lone pair exists. And thus there are repulsion forces possible in between the lone pair and bond pair of electrons thus the arrangement of electron pairs around both the molecules is same or different depending up on the conditions leading to maximum repulsion. 


