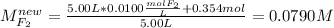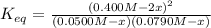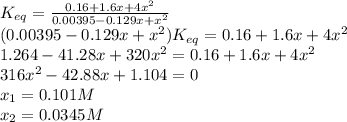Chemistry, 01.11.2019 02:31 krandall232
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in a 5.00 l rigid container are [h2] = 0.0500 m, [f2] = 0.0100 m, and [hf] = 0.400 m. if 0.345 mol of f2 is added to this equilibrium mixture, calculate the concentrations of all gases once equilibrium is reestablished in moles/liter. h2(g) + f2(g) < > 2 hf(g)

Answers: 2
Another question on Chemistry


Chemistry, 21.06.2019 23:30
Calculate the expected ph values of the buffer systems from the experiments (a,b,c,d), using the henderson- hasselbalch equation, ph-pka+log[a-]/[ha]. use for pka values carbonic acid= 6.37, and acetic acid= 4.75.
Answers: 2

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
For the reaction below at a certain temperature, it is found that the equilibrium concentrations in...
Questions



Biology, 04.07.2019 01:20







Medicine, 04.07.2019 01:20

Mathematics, 04.07.2019 01:20


Mathematics, 04.07.2019 01:20

Spanish, 04.07.2019 01:20




History, 04.07.2019 01:20


![[HF]_{eq}=0.469M, [H_2]_{eq}=0.0155M, [F_2]_{eq}=0.0445M](/tpl/images/0355/0697/87639.png)
![K_{eq}=\frac{[HF]^2}{[H_2][F_2]}=\frac{(0.400M)^2}{(0.0500M)(0.0100M)} =320](/tpl/images/0355/0697/ba1f2.png)