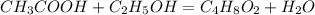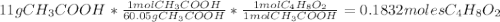Determine the theoretical maximum moles of ethyl acetate, , that could be produced in this experiment. the reactant, acetic acid, is the limiting reagent. (to avoid introducing rounding errors on intermediate calculations, enter your answer to four significant figures.) theoretical maximum moles of ethyl acetate = mol reactant mass 11.0 g product mass 10.8 g reactant moles 0.1833 mol

Answers: 3
Another question on Chemistry

Chemistry, 22.06.2019 00:30
Asolution of sodium hydroxide was titrated against a solution of sulfuric acid. how many moles of sodium hydroxide would react with 1 mole of sulfuric acid?
Answers: 2

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 05:40
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3
Answers: 3
You know the right answer?
Determine the theoretical maximum moles of ethyl acetate, , that could be produced in this experimen...
Questions













Business, 18.03.2021 01:50







Mathematics, 18.03.2021 01:50

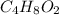 )
)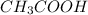 reacts with ethanol to produce ethyl acetate
reacts with ethanol to produce ethyl acetate