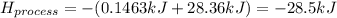Chemistry, 01.11.2019 02:31 tintlemax6256
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0.50 mol naoh at 22.5°c in a calorimeter, the temperature of the solution increases to 26.0°c. how much heat (in kj) was released by this reaction? note: the specific heat of water (cwater) is 4.18 j/(g•˚c) and the density of the solution is 1.00 g/ml.

Answers: 3
Another question on Chemistry


Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 16:50
What is conserved in the reaction shown below? h2(g) + cl2 (g) --> 2hcl(g)a. mass onlyb. mass and moles onlyc. mass, moles, and molecules onlyd. mass, moles, molecules, and volume
Answers: 2

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3
You know the right answer?
When 50.0 ml of water containing 0.50 mol hcl at 22.5°c are mixed with 50.0 ml of water containing 0...
Questions




Computers and Technology, 03.08.2019 07:00



Geography, 03.08.2019 07:00

English, 03.08.2019 07:00

Health, 03.08.2019 07:00



English, 03.08.2019 07:00

Mathematics, 03.08.2019 07:00

English, 03.08.2019 07:00

History, 03.08.2019 07:00

History, 03.08.2019 07:00

Health, 03.08.2019 07:00


Mathematics, 03.08.2019 07:00

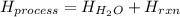
![H_{H_2O}=[(50.0mL+50mL)*\frac{1g}{1mL}]*4.18\frac{J}{mol^0C}*(26.0-22.5)^0C\\H_{H_2O}=146.3J=0.1463kJ](/tpl/images/0355/0596/d18d3.png)