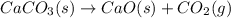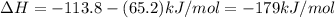Chemistry, 01.11.2019 02:31 blondielocks2002
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) + co₂(g) given the thermochemical equations below:
ca(oh)₂(s) --> cao(s) + h₂o(l); enthalpy reaction = 65.2 kj/mol
ca(oh)₂(s) + co₂(g) --> caco₃(s) + h₂o(l); enthalpy reaction = -113.8 kj/mol
c(s) + o₂(g) --> co₂(g); enthalpy of reation = -393.5 kj/mol
2ca(s) + o₂(g) --> 2cao(s); enthalpy of reaction = -1270.2 kj/mol
a. 1711.7 kj/mol rxn
b. 441 kj/mol rxn
c. 179 kj/mol rxn
d. 48 kj/mol rxn
e. 345.5 kj. mol rxn

Answers: 3
Another question on Chemistry

Chemistry, 21.06.2019 21:40
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1

You know the right answer?
Determine the enthalpy change for the decomposition of calcium carbonate. caco₃(s) --> cao(s) +...
Questions

History, 06.09.2019 20:30

Mathematics, 06.09.2019 20:30




Biology, 06.09.2019 20:30

History, 06.09.2019 20:30





English, 06.09.2019 20:30


English, 06.09.2019 20:30

Chemistry, 06.09.2019 20:30

English, 06.09.2019 20:30

Mathematics, 06.09.2019 20:30

Mathematics, 06.09.2019 20:30


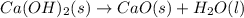
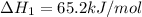 (1)
(1)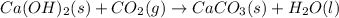
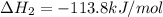 (2)
(2)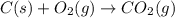
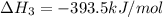 (3)
(3)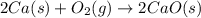
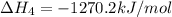 (4)
(4)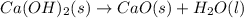 -
-