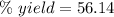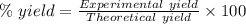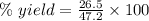Chemistry, 01.11.2019 03:31 tamikagoss22
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4, the theoretical yield in an experiment is calculated to be 47.2 g fe. when a careless chemistry student carries out the experiment, the actual yield is 26.5 g fe. calculate the percentage yield. chemical equation and the starting amounts of the reactants.

Answers: 2
Another question on Chemistry

Chemistry, 21.06.2019 13:00
Note the ph and poh values labeled with letters on the ph scale below. based on log rules and the way ph is calculated, what is the difference in [oh– ] concentration between point a and point b. a) 10^1 b) 10^5 c) 10^6 d) 10^7
Answers: 1

Chemistry, 22.06.2019 09:00
What term is missing from the central region that describes hypotheses, theories, and laws? popular predictable mathematical falsifiable
Answers: 2


Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1
You know the right answer?
The theoretical yield of a reaction can be determined using its in the reaction between co and fe3o4...
Questions


Mathematics, 24.04.2020 10:41


Mathematics, 24.04.2020 10:41

Physics, 24.04.2020 10:41


Mathematics, 24.04.2020 10:42

Mathematics, 24.04.2020 10:43

History, 24.04.2020 10:43

Mathematics, 24.04.2020 10:43

Mathematics, 24.04.2020 10:43

Chemistry, 24.04.2020 10:43

Mathematics, 24.04.2020 10:43

Geography, 24.04.2020 10:44

Chemistry, 24.04.2020 10:44

Mathematics, 24.04.2020 10:44

English, 24.04.2020 10:44



Mathematics, 24.04.2020 10:44